Iron Nitride Charge . roman numeral notation indicates charge of ion when element commonly forms more than one ion. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states). For example, iron(ii) has a 2+ charge;. iron can form two possible ions, but the ion with a 3+ charge is specified here. incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. Some elements exhibit a regular. this further shortened the diffusion length and sustained structural integrity during the pseudocapacitive charge. The oxide has a 2− charge as an ion. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge.
from pubs.rsc.org
this further shortened the diffusion length and sustained structural integrity during the pseudocapacitive charge. For example, iron(ii) has a 2+ charge;. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. roman numeral notation indicates charge of ion when element commonly forms more than one ion. incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. Some elements exhibit a regular. iron can form two possible ions, but the ion with a 3+ charge is specified here. The oxide has a 2− charge as an ion. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states).
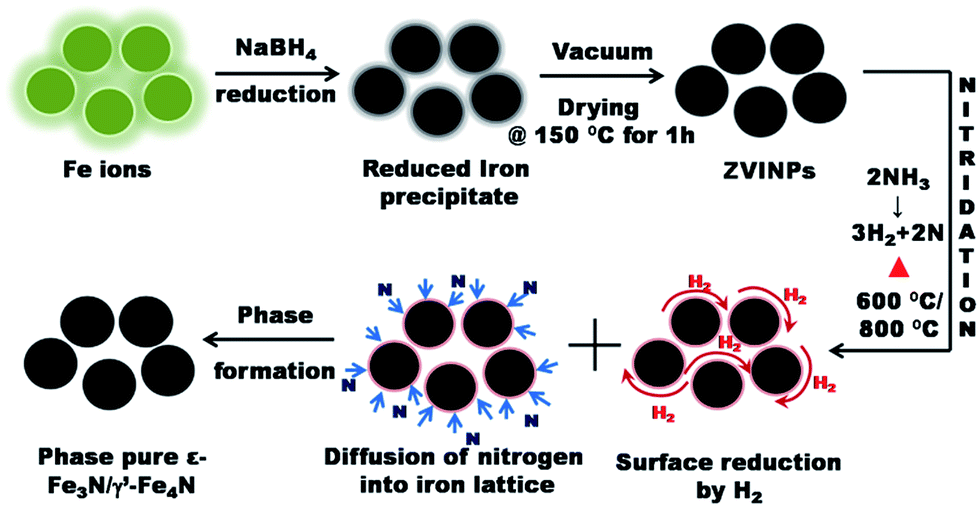
Insights into the nitridation of zerovalent iron nanoparticles for the
Iron Nitride Charge For example, iron(ii) has a 2+ charge;. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states). Some elements exhibit a regular. iron can form two possible ions, but the ion with a 3+ charge is specified here. The oxide has a 2− charge as an ion. For example, iron(ii) has a 2+ charge;. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. this further shortened the diffusion length and sustained structural integrity during the pseudocapacitive charge. incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. roman numeral notation indicates charge of ion when element commonly forms more than one ion.
From www.pinterest.com
N 3 Electron Configuration (Nitride Ion) Electron configuration Iron Nitride Charge Some elements exhibit a regular. The oxide has a 2− charge as an ion. incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. this further shortened the diffusion length and sustained structural integrity during the pseudocapacitive charge. roman numeral notation indicates charge of ion when element commonly forms more. Iron Nitride Charge.
From pubs.acs.org
Iron Nitride Thin Films Growth, Structure, and Properties Crystal Iron Nitride Charge iron can form two possible ions, but the ion with a 3+ charge is specified here. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states). For example, iron(ii) has a 2+ charge;. roman numeral notation indicates charge of ion when element commonly. Iron Nitride Charge.
From www.researchgate.net
Theoretically calculated highpressure phase diagrams of iron nitrides Iron Nitride Charge roman numeral notation indicates charge of ion when element commonly forms more than one ion. The oxide has a 2− charge as an ion. this further shortened the diffusion length and sustained structural integrity during the pseudocapacitive charge. For example, iron(ii) has a 2+ charge;. all the remaining transition metals form multiple charged ions (iron for example,. Iron Nitride Charge.
From www.researchgate.net
XRD pattern of carboncoated iron nitride nanoparticles synthesized at Iron Nitride Charge incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. roman numeral notation indicates charge of ion when element commonly forms more than one ion. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. Some elements. Iron Nitride Charge.
From www.researchgate.net
Atomic arrangement of and iron nitride (Fe4N) a. Crystal Iron Nitride Charge The oxide has a 2− charge as an ion. incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states). For example, iron(ii) has a 2+ charge;. . Iron Nitride Charge.
From hackaday.com
Iron Nitrides Powerful Without The Rare Earth Elements Hackaday Iron Nitride Charge for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. The oxide has a 2− charge as an ion. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states). iron. Iron Nitride Charge.
From www.researchgate.net
Thickness and the electric resistivity of the iron nitride films Iron Nitride Charge for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. For example, iron(ii) has a 2+ charge;. iron can form two possible ions, but the ion with a 3+ charge is specified here. this further shortened the diffusion length and sustained structural integrity during. Iron Nitride Charge.
From pubs.acs.org
Iron Nitride Thin Films Growth, Structure, and Properties Crystal Iron Nitride Charge for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. iron can form two possible ions, but the ion with a 3+ charge is specified here. For. Iron Nitride Charge.
From pubs.acs.org
Iron Nitride Nanoparticles for Enhanced Reductive Dechlorination of Iron Nitride Charge The oxide has a 2− charge as an ion. roman numeral notation indicates charge of ion when element commonly forms more than one ion. For example, iron(ii) has a 2+ charge;. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states). incorporating an. Iron Nitride Charge.
From pubs.acs.org
Iron Nitride Thin Films Growth, Structure, and Properties Crystal Iron Nitride Charge iron can form two possible ions, but the ion with a 3+ charge is specified here. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states). this further shortened the diffusion length and sustained structural integrity during the pseudocapacitive charge. incorporating an. Iron Nitride Charge.
From pubs.rsc.org
Insights into the nitridation of zerovalent iron nanoparticles for the Iron Nitride Charge iron can form two possible ions, but the ion with a 3+ charge is specified here. incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. The oxide has a 2− charge as an ion. this further shortened the diffusion length and sustained structural integrity during the pseudocapacitive charge. . Iron Nitride Charge.
From www.researchgate.net
(PDF) Formation of α″ iron nitride in FeN martensite Nitrogen Iron Nitride Charge incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. Some elements exhibit a regular. For example, iron(ii) has a 2+ charge;. this further shortened the diffusion length and sustained structural integrity during the pseudocapacitive charge. iron can form two possible ions, but the ion with a 3+ charge is. Iron Nitride Charge.
From pubs.acs.org
NobleMetalFree Iron Nitride/NitrogenDoped Graphene Composite for the Iron Nitride Charge The oxide has a 2− charge as an ion. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states). this further shortened the diffusion length and sustained structural integrity during the pseudocapacitive charge. iron can form two possible ions, but the ion with. Iron Nitride Charge.
From www.youtube.com
N 3 Electron Configuration (Nitride Ion) YouTube Iron Nitride Charge incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. iron can form two possible ions, but the ion with a 3+ charge is specified here. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states).. Iron Nitride Charge.
From pubs.acs.org
Iron Nitride Thin Films Growth, Structure, and Properties Crystal Iron Nitride Charge for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states). roman numeral notation indicates charge of ion when element commonly. Iron Nitride Charge.
From www.semanticscholar.org
[PDF] Structure of iron nitrides under different nitridation Iron Nitride Charge For example, iron(ii) has a 2+ charge;. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a 2+ or 3+ charge. this further shortened the diffusion length and sustained structural integrity during the pseudocapacitive charge. roman numeral notation indicates charge of ion when element commonly forms more than. Iron Nitride Charge.
From pubs.rsc.org
Stepwise NH bond formation from N 2 derived iron nitride, imide and Iron Nitride Charge iron can form two possible ions, but the ion with a 3+ charge is specified here. For example, iron(ii) has a 2+ charge;. all the remaining transition metals form multiple charged ions (iron for example, forms fe +2 and fe +3 and thus has multiple charge states). roman numeral notation indicates charge of ion when element commonly. Iron Nitride Charge.
From www.researchgate.net
XRD pattern of synthesized iron nitride samples at different plasma Iron Nitride Charge Some elements exhibit a regular. iron can form two possible ions, but the ion with a 3+ charge is specified here. incorporating an alkali metal into an otherwise metallic binary nitride can increase charge localization driven by the. for example, copper can form ions with a 1+ or 2+ charge, and iron can form ions with a. Iron Nitride Charge.